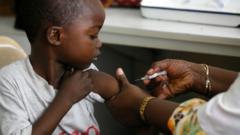
The medical community has celebrated a pivotal moment in the fight against malaria with the recent approval of the first treatment intended specifically for infants and very young children. This groundbreaking treatment is anticipated to be implemented in various African nations within weeks, targeting a demographic that has been historically underserved by existing therapies. Previously, babies suffering from malaria were treated with formulations designed for older children, putting them at heightened risk of overdose due to the differences in their physiological responses to medications.
The latest data indicates that malaria was responsible for approximately 597,000 deaths in 2023, with a staggering majority occurring in Africa, particularly among children under the age of five. The urgency for a specifically tailored treatment for this age group has long been recognized by health experts, who have expressed concern over the gap in appropriate medical solutions for infants.
Developed by Novartis, the newly approved medication, known as Coartem Baby (or Riamet Baby in certain regions), has garnered approval from Swiss health authorities and is expected to be distributed to regions with the highest malaria prevalence shortly. This initiative is set to be largely non-profit, ensuring expanded access to this critical medication.
Novartis Chief Executive Vas Narasimhan emphasized the significance of this milestone, stating, "Together with our partners, we are proud to have gone further to develop the first clinically proven malaria treatment for newborns and young babies." The treatment was developed alongside the Medicines for Malaria Venture (MMV), a non-profit organization supported by various governments and foundations.
Commenting on this progress, Martin Fitchet, CEO of MMV, highlighted the urgent need to address the high mortality rates associated with malaria, particularly in young children. "With the right resources and focus, it can be eliminated," he said.
Furthermore, Dr. Marvelle Brown from the University of Hertfordshire acknowledged the breakthrough's potential impact on reducing child mortality rates from malaria, particularly in sub-Saharan Africa, where the disease disproportionately affects this demographic.
As the healthcare community anticipates the rollout of this innovative treatment, it represents a vital step towards improving healthcare equity and protecting some of the most vulnerable populations against one of the world’s deadliest diseases.