Women using the affected pills are advised to seek medical consultation immediately.
Recall Alert: Yaz Plus Contraceptive Pill Issues in South Africa
Recall Alert: Yaz Plus Contraceptive Pill Issues in South Africa
Bayer Ltd. recalls a specific batch of Yaz Plus due to incorrect packaging, posing a risk of ineffective contraception.
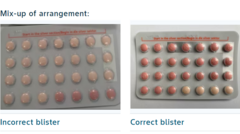
The South African health authorities have issued a recall for a particular batch of the widely used Yaz Plus contraceptive pill following a serious packaging error. The mix-up involved blister packs that incorrectly contained 24 inactive pills instead of the correct 24 active hormone pills, significantly compromising the product’s effectiveness.
Bayer Ltd., the manufacturer of Yaz Plus, has instructed women who may have ingested pills from the compromised batch, labeled as WEW96J and set to expire in March 2026, to stop taking them immediately and consult healthcare professionals. This precautionary measure comes from a clear understanding that consuming these inactive pills could lead to unintended pregnancies, as users might mistakenly believe they are taking effective hormonal contraception.
The Yaz Plus packaging typically consists of 24 pink active pills containing hormones followed by four light orange inactive pills. However, the affected batch contained only 24 light orange inactive pills, with a mere four active hormone pills. Bayer has confirmed that they have resolved the root cause of this issue and collaborated closely with the South African Health Products Regulatory Agency to address the recall.
The company reported that, while the recall affects a limited number of packs, they recommend returning any pills from the specific batch to pharmacies for either a refund or replacement. Healthcare workers, including pharmacies, hospitals, and nurses, are also asked to return any remaining packages from the faulty batch.
To support those impacted by this recall, Bayer Ltd. has established a helpline, assuring customers that the incident is confined to a single batch, with no other Yaz Plus products being implicated in this mix-up. The health and safety of users remain the top priority for Bayer as they navigate this issue.
Bayer Ltd., the manufacturer of Yaz Plus, has instructed women who may have ingested pills from the compromised batch, labeled as WEW96J and set to expire in March 2026, to stop taking them immediately and consult healthcare professionals. This precautionary measure comes from a clear understanding that consuming these inactive pills could lead to unintended pregnancies, as users might mistakenly believe they are taking effective hormonal contraception.
The Yaz Plus packaging typically consists of 24 pink active pills containing hormones followed by four light orange inactive pills. However, the affected batch contained only 24 light orange inactive pills, with a mere four active hormone pills. Bayer has confirmed that they have resolved the root cause of this issue and collaborated closely with the South African Health Products Regulatory Agency to address the recall.
The company reported that, while the recall affects a limited number of packs, they recommend returning any pills from the specific batch to pharmacies for either a refund or replacement. Healthcare workers, including pharmacies, hospitals, and nurses, are also asked to return any remaining packages from the faulty batch.
To support those impacted by this recall, Bayer Ltd. has established a helpline, assuring customers that the incident is confined to a single batch, with no other Yaz Plus products being implicated in this mix-up. The health and safety of users remain the top priority for Bayer as they navigate this issue.


















