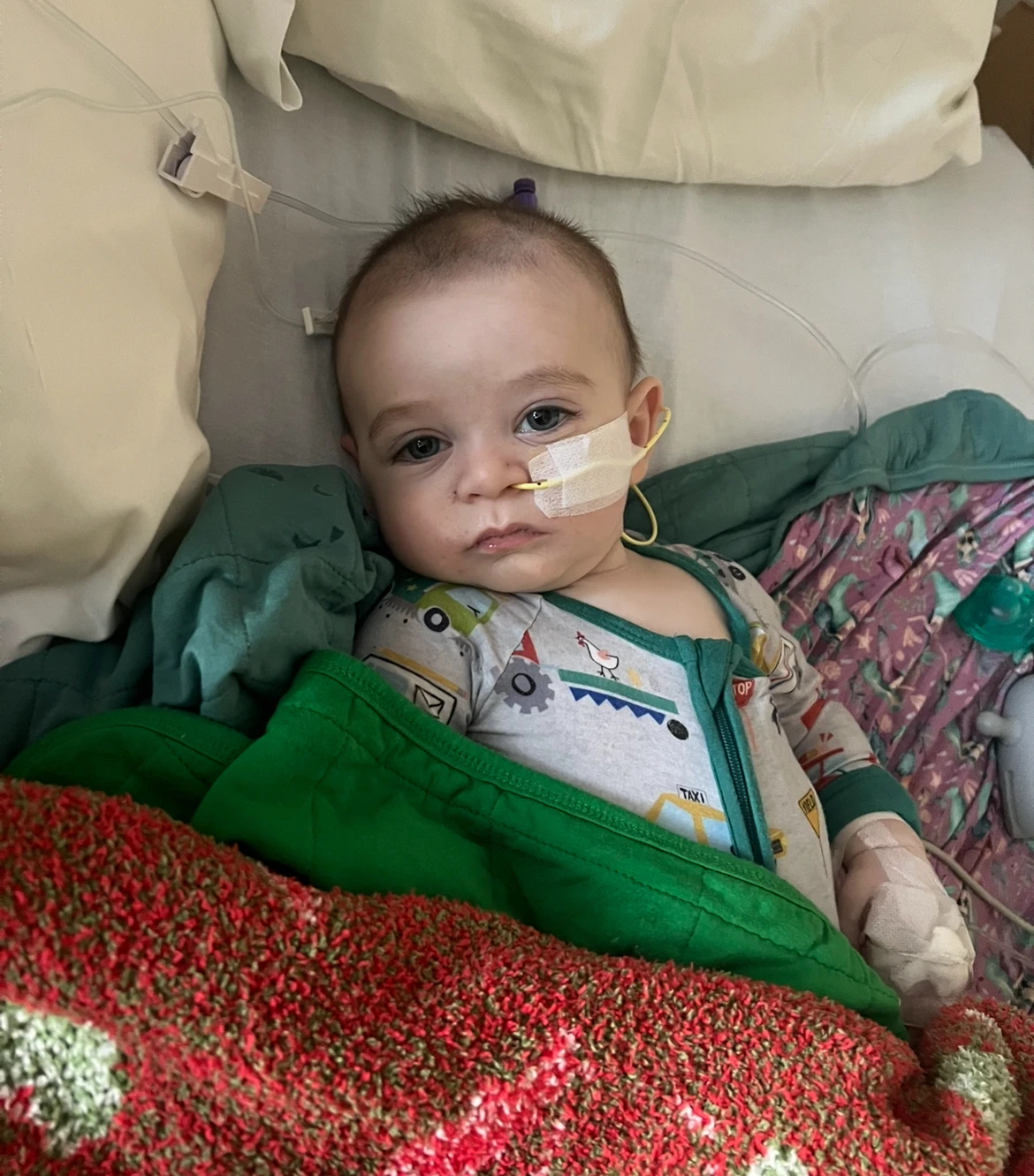
The parents of two infants hospitalized due to an outbreak of infantile botulism are suing the makers of the ByHeart baby formula, which is at the center of a nationwide recall. Stephen and Yurany Dexter of Flagstaff, Arizona, allege that their 4-month-old daughter Rose required a helicopter transport to a children’s hospital for life-saving treatment this summer. Similarly, Michael and Hanna Everett from Richmond, Kentucky, reported that their daughter Piper was also rushed to the hospital with severe symptoms related to the illness.
Both lawsuits were filed in federal courts and claim that ByHeart’s formula was defective and that the company acted negligently. The families are seeking financial compensation not only for medical bills but also for the emotional distress caused by their children’s serious health crises.
I wouldn’t have expected a product created for vulnerable infants in the U.S. to cause something so severe, Stephen Dexter expressed, reflecting the shock many parents feel about the incident.
Outbreak Timeline and Response
Rose Dexter and Piper Everett are among at least 15 infants across multiple states affected by the outbreak that officials reported began in August. Fortunately, there have been no fatalities associated with this outbreak. Both infants received the only treatment available for botulism in young children, known as BabyBIG, which is derived from blood plasma of immunized donors.
Following the nationwide recall of ByHeart formula, investigations into other potential cases are ongoing. The New York-based manufacturer claims to sell approximately 200,000 cans of formula per month and was notified of the contamination risks after a sample from an open can linked to an infant's illness tested positive for botulism bacteria.
The lawsuits may indicate the beginning of a broader legal response against ByHeart, as food safety experts warn that the company faces significant repercussions. ByHeart has not publicly responded to the individual lawsuits but has stated its commitment to addressing any legal issues that arise.
A Deepening Concern for Affected Families
The Dexter family reported using ByHeart’s formula shortly after Rose's birth in July when insufficient breast milk prompted them to seek alternatives. Despite choosing the organic product, concerns arose when Rose exhibited serious health issues. After multiple warning signs and a dramatic decline in her health, they sought emergency care.
Meanwhile, the Everett family utilized ByHeart formula to supplement breastfeeding. They also experienced a troubling sequence of events when Piper's health rapidly deteriorated, forcing an emergency visit to the hospital after discovering the recalled product had been used.
Fortunately, both infants have shown signs of recovery after receiving the necessary medical treatment and are now doing well on alternative formulas.