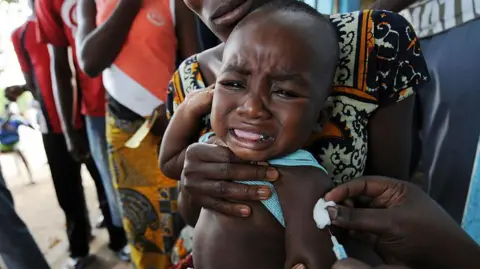
A now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau has been criticized by the World Health Organization (WHO) as unethical.
The US-funded study had sought to give one set of babies the vaccine at birth, while another would have had the shot delayed until six weeks of age. The WHO stated it had significant concerns about the plan, describing the birth-dose vaccine as an effective and essential public health intervention, with a proven record.
The trial's backlash comes amid a broader vaccine skepticism fueled by US Health Secretary Robert F. Kennedy Jr.’s controversial questioning of vaccine impacts. The WHO pointed out that the jab has been used globally for over three decades in more than 115 countries and emphasized that withholding it exposes some newborns to potentially irreversible harm.
The trial intended to involve 14,000 babies but has faced public outrage that led to its suspension. Critics highlight ethical concerns regarding the selection of Guinea-Bissau’s newborns for such studies where proven treatments already exist. The WHO has pushed for all newborns to receive the hepatitis B vaccine within 24 hours of birth, citing a high prevalence of the virus in Guinea-Bissau, where about 12% of the adult population lives with chronic Hepatitis B.
Although the dose is currently administered at six weeks, local authorities plan to introduce the birth dose nationwide by 2028, an effort the WHO supports. Critics, including former Guinea-Bissau health minister Magda Robalo, have strongly opposed the trial, emphasizing that Guinea-Bissauans should not be viewed as guinea pigs for experimental studies.
The US-funded study had sought to give one set of babies the vaccine at birth, while another would have had the shot delayed until six weeks of age. The WHO stated it had significant concerns about the plan, describing the birth-dose vaccine as an effective and essential public health intervention, with a proven record.
The trial's backlash comes amid a broader vaccine skepticism fueled by US Health Secretary Robert F. Kennedy Jr.’s controversial questioning of vaccine impacts. The WHO pointed out that the jab has been used globally for over three decades in more than 115 countries and emphasized that withholding it exposes some newborns to potentially irreversible harm.
The trial intended to involve 14,000 babies but has faced public outrage that led to its suspension. Critics highlight ethical concerns regarding the selection of Guinea-Bissau’s newborns for such studies where proven treatments already exist. The WHO has pushed for all newborns to receive the hepatitis B vaccine within 24 hours of birth, citing a high prevalence of the virus in Guinea-Bissau, where about 12% of the adult population lives with chronic Hepatitis B.
Although the dose is currently administered at six weeks, local authorities plan to introduce the birth dose nationwide by 2028, an effort the WHO supports. Critics, including former Guinea-Bissau health minister Magda Robalo, have strongly opposed the trial, emphasizing that Guinea-Bissauans should not be viewed as guinea pigs for experimental studies.